This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 描述 |
Protoporphyrin IX是血红素生物合成途径中的最终中间体。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 靶点 |
Human Endogenous Metabolite
|
||||||||||||
| 溶解度 |
体外:
DMSO:6.4 mg / mL(11.37 mM;需要超声波和加热)
|
||||||||||||
| 储备液 |
|
||||||||||||
| 存储 |
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
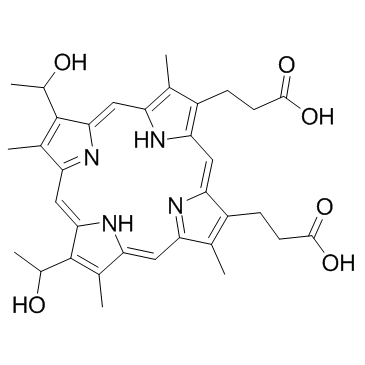
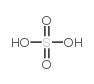
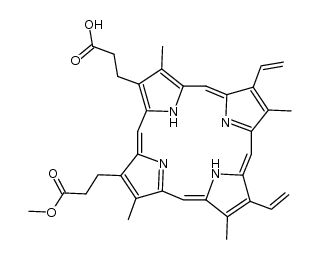
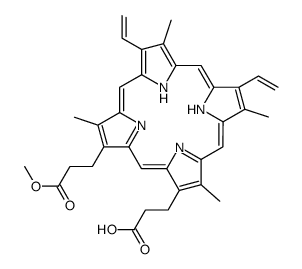
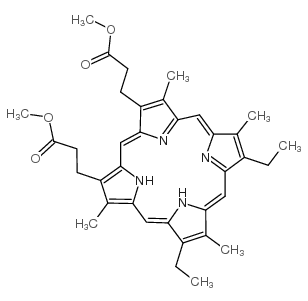
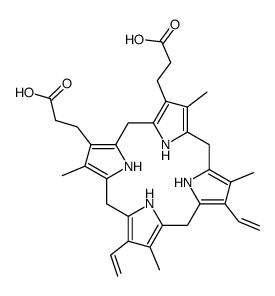
O=C(O)CCC1=C2/C=C3C(CCC(O)=O)=C(C)C(/C=C(N/4)/C(C)=C(C=C)C4=CC5=N/C(C(C=C)=C5C)=CC(N2)=C1C)=N/3 |
||||||||||||
| 参考文献 |
|
||||||||||||
| 相关活性 小分子 |
6-氨基-3-甲基嘌呤 | 氢化可的松 | N-乙酰-L-半胱氨酸 | 维A酸 | 褪黑素 | 地诺前列酮 | 烟酰胺 | 5′-三磷酸腺苷 | N-乙酰对氨基酚 | 列腺素 E1 | 去氢表雄酮 | 皮质酮 | 黄体酮 | 顺式-4,7,10,13,16,19-二十二碳六烯酸 | 辅酶I |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 1122.0±65.0 °C at 760 mmHg |
| 分子式 | C34H34N4O4 |
| 分子量 | 562.658 |
| 闪点 | 632.4±34.3 °C |
| 精确质量 | 562.257996 |
| PSA | 130.90000 |
| LogP | 7.33 |
| 外观性状 | Powder | purple |
| 蒸汽压 | 0.0±0.3 mmHg at 25°C |
| 折射率 | 1.674 |
| 储存条件 |
2-8?C |
| 分子结构 |
1、 摩尔折射率:162.60 2、 摩尔体积(m3/mol):433.2 3、 等张比容(90.2K):1233.4 4、 表面张力(dyne/cm):65.7 5、 极化率(10-24cm3):64.46 |
| 计算化学 |
1.疏水参数计算参考值(XlogP):4.6 2.氢键供体数量:4 3.氢键受体数量:6 4.可旋转化学键数量:8 5.互变异构体数量:1001 6.拓扑分子极性表面积132 7.重原子数量:42 8.表面电荷:0 9.复杂度:1010 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 |
1. 性状:棕黄色棱柱状结晶。对光和空气敏感。 2. 密度(g/mL,25/4℃): 3. 相对蒸汽密度(g/mL,空气=1): 4. 熔点(?C): 5. 沸点(?C,常压): 6. 沸点(?C,5.2kPa): 7. 折射率: 8. 闪点(?C): 9. 比旋光度(?): 10. 自燃点或引燃温度(?C): 11. 蒸气压(kPa,25?C): 12. 饱和蒸气压(kPa,60?C): 13. 燃烧热(KJ/mol): 14. 临界温度(?C): 15. 临界压力(KPa): 16. 油水(辛醇/水)分配系数的对数值: 17. 爆炸上限(%,V/V): 18. 爆炸下限(%,V/V): 19. 溶解性:易溶于氯仿、冰乙酸及含有盐酸的乙醇、含有冰乙酸、盐酸的乙醚,微溶于稀碱、苯胺和吡啶。 |
| 符号 |

GHS07 |
|---|---|
| 信号词 |
Warning |
| 危害声明 |
H315-H319-H335 |
| 警示性声明 |
P280-P305 + P351 + P338-P337 + P313 |
| 个人防护装备 |
dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) |
Xi |
| 风险声明 (欧洲) |
R36/37/38 |
| 安全声明 (欧洲) |
S26;S36 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| 原卟啉上游产品? 6 | |
|---|---|
|
|
|
|
| 原卟啉下游产品? 10 | |
|
|
|
|
|
|
|
|
|
Retinoic acid synergizes ATO-mediated cytotoxicity by precluding Nrf2 activity in AML cells. Br. J. Cancer 111(5) , 874-82, (2014)
Standard therapy for acute promyelocytic leukaemia (APL) includes retinoic acid (all-trans retinoic acid (ATRA)), which promotes differentiation of promyelocytic blasts. Although co-administration of …
|
|
|
Indomethacin inhibits activation of endothelial nitric oxide synthase in the rat kidney: possible role of this effect in the pathogenesis of indomethacin-induced renal damage. Chem. Biol. Interact. 221 , 77-87, (2014)
The clinical use of non-steroidal anti-inflammatory drugs (NSAIDs) is often associated with adverse effects in the kidney. Indomethacin, an NSAID that has been shown to induce oxidative stress in the …
|
|
|
Comparison of protoporphyrin IX produced cell proliferation inhibition between human breast cancer MCF-7 and MDA-MB-231 cells. Pharmazie 69(8) , 621-8, (2014)
Protoporphyrin IX (PpIX) is an effective hematoporphyrin derivative, widely adopted in photodynamic therapy (PDT) and sonodynamic therapy (SDT). As a sensitizer, PpIX could significantly enhance laser…
|
|
|
3,3′-(3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphyrindiyl)dipropanoic acid |
|
3,3′-(7,12-diethenyl-3,8,13,17-tetramethylporphyrin-2,18-diyl)dipropanoic acid |
|
Kammerer’s prophyrin |
|
1,3,5,8-Tetramethyl-2,4-divinylporphine-6,7-dipropionic Acid |
|
MFCD00151109 |
|
3,3′-(3,7,12,17-Tetramethyl-8,13-divinylporphyrin-2,18-diyl)dipropanoic acid |
|
21H,23H-Porphine-2,18-dipropanoic acid, 7,12-diethenyl-3,8,13,17-tetramethyl- |
|
protoporphyrin |
|
EINECS 209-033-7 |
|
Kammerer’s porphyrin |
|
protoporphyrin-IX |
|
7,12-Diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoic acid |
|
Protoporphyrin IX |











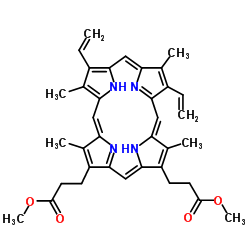
![[5,5'-diformyl-4,4'-dimethyl-3,3'-bis[2-(methoxycarbonyl)ethyl]-2,2'-dipyrryl]methane结构式](https://www.chemsrc.com/caspic/146/4792-10-3.png)