This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 描述 |
Procyanidin B2 是一种天然黄酮类物质,具有抗肿瘤,抗氧化等作用。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 体外研究 |
原花青素B2显示对MCF-7细胞的抗增殖活性,IC50为19.21μM。然而,原花青素B2对DNA梯形成没有影响[1]。原花青素B2(0.1,1,2μM)抑制人脐静脉内皮细胞(HUVECs)中含有3(NLRP3)炎性体的pyrin结构域的激活,并且通过抑制AP-1活性来抑制这种作用,并且可以通过抑制这种作用来消除这种作用。过表达c-Jun。原花青素B2(2μM,12小时)也可降低HUVEC中的ROS [2]。 |
||||||||||||
| 体内研究 |
原花青素B2(40,20和10mg / kg,口服)保护免受脑缺血诱导的大鼠梗塞体积和脑水肿。原花青素B2(40mg / kg,口服)也改善功能结果,调节脑缺血后的血脑屏障(BBB)通透性。此外,原花青素B2减轻脑缺血诱导的紧密连接降解,线粒体去极化和细胞内氧化应激。原花青素B2(40 mg / kg,po)可增加体内正常大脑中Nrf2的活化和HO-1,GSTα和NQO1蛋白的表达[3]。 |
||||||||||||
| 溶解度 |
体外:
DMSO:≥50mg / mL(86.43 mM) * “≥”表示可溶,但饱和度未知。 体内:1。逐个添加每种溶剂:10%DMSO 90%玉米油溶解度:≥2.5mg / mL(4.32 mM);澄清溶液2.逐个加入各溶剂:10%DMSO 90%(20%SBE-β-CD在盐水中)溶解度:≥2.5mg/ mL(4.32mM);澄清溶液3.逐个添加每种溶剂:10%DMSO 40%PEG300 5%吐温-80 45%盐水溶解度:≥2.5mg/ mL(4.32mM);明确解决方案
|
||||||||||||
| 储备液 |
|
||||||||||||
| 细胞实验 |
使MCF-7细胞在5mL补充有20%胎牛血清,青霉素(100U / mL)和链霉素(100μg/ mL)的RPMI 1640培养基中生长。将细胞保持在37℃,5%CO 2的空气湿润气氛中,每3天传代培养。将指数生长的细胞以2×10 4个细胞/ mL的接种密度接种在96孔板或培养皿中。在孵育过夜以允许附着后,将细胞暴露24或48小时至在蒸馏水中稀释的各种浓度的原花青素B2(0.5; 1.0; 5.0; 10.0; 25.0和50.0μM)。使用2毫摩尔环磷酰胺(CP)作为阳性对照,并通过过滤对溶液进行灭菌[1]。 |
||||||||||||
| 动物实验 |
为了观察原花青素B2对梗死面积或脑水肿的影响,将40只大鼠随机分成5组,每组给予载体(通过管饲法施用相当剂量的0.9%盐水)或40,20或10mg /分别为kg的原花青素B2。为了阐明原花青素B2对血脑屏障(BBB)通透性的影响,大鼠(伊文思蓝外渗每组n = 6,IgG组泄漏每组n = 4)随机分为两组:载体和原花青素B2 (40毫克/千克)。从大脑中动脉闭塞(MCAO)后3小时开始,每天一次胃内施用原花青素B2(40mg / kg)。对于其他观察,包括免疫组织学染色和蛋白质印迹分析,将大鼠随机分成三组:假手术,载体和原花青素B2(每组n = 4)。从MCAO后3小时开始,每天一次胃内施用原花青素B2(40mg / kg)。为了阐明大鼠缺血性中风后神经功能的改善,大鼠(每组n = 8)进行神经行为测定以评估在MCAO后24小时开始每天一次施用原花青素B2(40mg / kg)后的功能结果。 。在MCAO后1,3,7,11和14天评估神经功能缺损[3]。 |
||||||||||||
| 存储 |
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
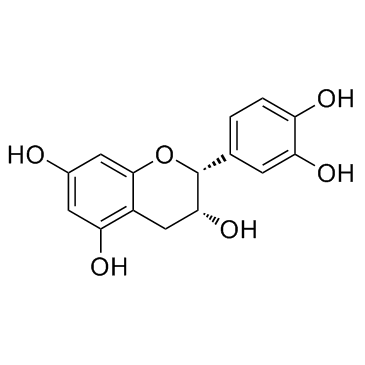
O[C@H]1[C@@H](C2=CC=C(O)C(O)=C2)OC3=CC(O)=CC(O)=C3[C@@H]1C4=C5C(C[C@@H](O)[C@@H](C6=CC=C(O)C(O)=C6)O5)=C(O)C=C4O |
||||||||||||
| 参考文献 |
|
||||||||||||
| 相关活性 小分子 |
磺丁醚-beta-环糊精 | 环孢菌素 | 2,7-二氯二氢代荧光黄二醋酸 | 1-甲基-4-苯基-1,2,3,6-四氢吡啶盐酸盐 | 4′,4”-双-2-咪唑啉-2-基对苯二丙烯酰苯胺二盐酸盐 | 乙莫克舍 | TD139 | Mitoquinone甲磺酸盐 | GSK2795039 | 5,5’,6,6’-四氯-1,1’,3,3’-四乙基苯并咪唑羰花青碘化物 | 胞内钙荧光探针 BAPTA-AM | AP20187 | GKT137831 | D-虫荧光素游离酸 | 野百合碱 |
| 密度 | 1.7±0.1 g/cm3 |
|---|---|
| 沸点 | 955.3±65.0 °C at 760 mmHg |
| 熔点 | 197-198?C |
| 分子式 | C30H26O12 |
| 分子量 | 578.520 |
| 闪点 | 531.6±34.3 °C |
| 精确质量 | 578.142456 |
| PSA | 220.76000 |
| LogP | 0.30 |
| 蒸汽压 | 0.0±0.3 mmHg at 25°C |
| 折射率 | 1.803 |
| 储存条件 | 2-8°C |
| 个人防护装备 |
Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 安全声明 (欧洲) |
22-24/25 |
| 危险品运输编码 | NONH for all modes of transport |
| 原花青素B2上游产品? 9 | |
|---|---|
|
|
|
|
|
|
|
| 原花青素B2下游产品? 3 | |
|
|
|
|
Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 161 , 79-86, (2014)
Antimicrobial properties of ethanol and water extracts from eight Asteraceae species were investigated against three Gram positive (Staphylococcus aureus, MRSA and Bacillus cereus) and two Gram negati...
|
|
|
UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 62(39) , 9387-400, (2014)
Oligomeric proanthocyanidins were successfully identified by UHPLC-PDA-HRMS(n) in a selection of plant-derived materials (jujube fruit, Fuji apple, fruit pericarps of litchi and mangosteen, dark choco...
|
|
|
A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 5(9) , 2357-64, (2014)
We have previously reported that procyanidins, a class of flavonoids, improve glycemia and exert an incretin-like effect, which was linked to their proven inhibitory effect on the dipeptidyl-peptidase...
|
|
|
Proanthocyanidin B3 |
|
Proanthocyanidin B2 |
|
(2R,2'R,3R,3'R,4R)-2,2'-Bis(3,4-dihydroxyphenyl)-3,3',4,4'-tetrahydro-2H,2'H-4,8'-bichromene-3,3',5,5',7,7'-hexol |
|
(2R,3S)-2-(3,4-dihydroxyphenyl)-8-[(2R,3S,4S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol |
|
4,8″-Bi-[(+)-epicatechin] |
|
PROCYANIDINDIMERB2 |
|
EPICATECHIN(4BETA->8)EPICATECHIN |
|
Procyanidol B2 |
|
(-)epicatechin-(-)-epicatechin |
|
Procyanidin B2 |
|
EPICATECHIN(4B-8)EPICATECHIN |
|
ProcyanidinB2 |
|
PROCYANIDIN B1(P) |
|
Procyanidin B-3 |
|
Proanthocyanidin |
|
UNII-L88HKE854X |
|
4,8′-BI-[(+)-EPICATECHIN] |
|
2,3-trans-proanthocyanidin |
|
[4,8'-Bi-2H-1-benzopyran]-3,3′,5,5′,7,7′-hexol, 2,2′-bis(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-, (2R,2′R,3R,3′R,4R)- |











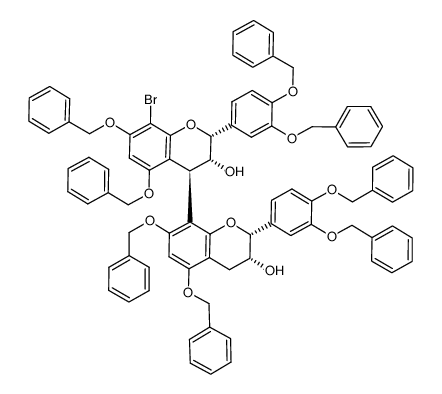
![5,7,3',4'-tetra-O-benzyl-(-)-epicatechin-(4β,8)-[5,7,3',4'-tetra-O-benzyl-(-)-epicatechin]结构式](https://www.chemsrc.com/caspic/027/223387-28-8.png)
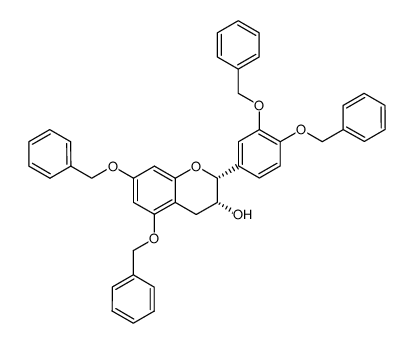
![(2R)-2-[3,4-bis(phenylmethoxy)phenyl]-5,7-bis(phenylmethoxy)-4H-chromen-3-one结构式](https://www.chemsrc.com/caspic/087/87292-54-4.png)