This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 中文名 |
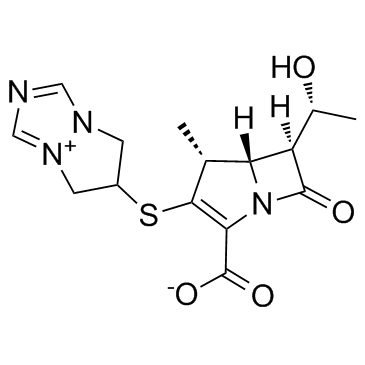
比阿培南 |
|---|---|
| 英文名 |
biapenem |
| 中文别名 |
比亚培南 | 6-[[(4R,5S,6S)-2-羧基-6-((1R)-1-羟乙基)-4-甲基-7-氧代-1-氮杂双环[3.2.0]庚-2-烯-3-基]硫]-6,7-双氢-5H-哌唑酮[1,2-a][1,2,4]三氮杂-4-内盐 | 比阿培南 |
| 英文别名 |
更多 |
| 描述 |
Biapenem是不经肠道的广谱碳青霉烯抗生素。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 溶解度 |
体外:
H 2 O:20 mg / mL(57.08 mM;需要超声波)
|
||||||||||||
| 储备液 |
|
||||||||||||
| 存储 |
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
O=C1N2C(C([O-])=O)=C(SC(C3)CN4[N+]3=CN=C4)[C@H](C)[C@]2([H])[C@@]1([H])[C@H](O)C |
||||||||||||
| 参考文献 |
[1]. Perry, C.M. and T. Ibbotson, Biapenem. Drugs, 2002. 62(15): p. 2221-34; discussion 2235.
|
||||||||||||
| 相关活性 小分子 |
嘌呤霉素盐酸盐 | G-418 硫酸盐 | 衣霉素 | 潮霉素B | 盐霉素 | 阿维巴坦钠 | 硫酸新霉素 | 法硼巴坦 | 甲氧西林钠 | 利福平 | 甲硝唑 | 羧苄青霉素钠 | 头孢他啶 | Eravacycline dihydrochloride | 头孢噻肟钠 |
| 熔点 | 265-271°C (dec.) |
|---|---|
| 分子式 | C15H18N4O4S |
| 分子量 | 350.393 |
| 精确质量 | 350.104889 |
| PSA | 127.67000 |
| 外观性状 | 白色至淡黄色粉末 |
| 折射率 | 1.651 |
| 储存条件 | -20?C Freezer |
| 危险品运输编码 | NONH for all modes of transport |
|---|
|
~%
比阿培南
120410-24-4
|
|
文献:Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 – 4249 |
|
~%

比阿培南
120410-24-4
|
|
文献:Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 – 4249 |
|
~%

比阿培南
120410-24-4
|
|
文献:Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 – 4249 |
|
Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2010: General view of the pathogens’ antibacterial susceptibility. J. Infect. Chemother. 21 , 410-20, (2015)
The nationwide surveillance on antimicrobial susceptibility of bacterial respiratory pathogens from patients in Japan, was conducted by Japanese Society of Chemotherapy, Japanese Association for Infec…
|
|
|
Novel approach to optimize synergistic carbapenem-aminoglycoside combinations against carbapenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 59 , 2286-98, (2015)
Acinetobacter baumannii is among the most dangerous pathogens and emergence of resistance is highly problematic. Our objective was to identify and rationally optimize β-lactam-plus-aminoglycoside comb…
|
|
|
Biapenem inactivation by B2 metallo β-lactamases: energy landscape of the post-hydrolysis reactions. PLoS ONE 7(1) , e30079, (2012)
The first line of defense by bacteria against β-lactam antibiotics is the expression of β-lactamases, which cleave the amide bond of the β-lactam ring. In the reaction of biapenem inactivation by B2 m…
|
|
|
Biapenem (JAN/USAN) |
|
EINECS 204-352-8 |
|
5H-Pyrazolo[1,2-a][1,2,4]triazol-4-ium, 6-[[(4R,5S,6S)-2-carboxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-, inner salt |
|
BiapeneM Crude |
|
Biapenem [USAN:INN] |
|
(4R,5S,6S)-3-(6,7-Dihydro-5H-pyrazolo[1,2-a][1,2,4]triazol-4-ium-6-ylsulfanyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate |
|
[4R-[4a,5b,6b(R*)]]-6-[[2-Carboxy-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5 H-pyrazolo[1,2-a][1,2,4]triazol-4-ium inner salt |
|
LJ C10627 |
|
omegacin |
|
MFCD00864863 |
|
Biapenem |
|
LJ C10,627 |
|
Biapenern |
|
L-627 |











![4-nitrobenzyl (4R,5R,6S)-6-[(1R)-1-hydroxyethyl]-4-methyl-3,7-dioxo-1-azabicyclo[3.2.0]-heptane-2-carboxylate结构式](https://www.chemsrc.com/caspic/055/104873-15-6.png)
![p-Nitrobenzyl (1R,5S,6S)-2-[[(N,N-Bis(p-nitrobenzyloxycarbonyl)pyrazolidin-4-yl]thio]-6-[(1R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylate结构式](https://www.chemsrc.com/caspic/071/120764-68-3.png)