This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 中文名 |
洛匹那韦 |
|---|---|
| 英文名 |
lopinavir |
| 中文别名 |
洛匹那韦 LOPINAVIR | (2S)-N-[(2R,4S,5S)-5-[[2-(2,6-二甲基苯氧基)乙酰]氨基]-4-羟基-1,6-二苯基-己-2-基]-3-甲基-2-(2-氧代-1,3-二氮杂环己-1-基)丁酰胺 | 洛吡那韦 | 罗平拉韦 |
| 英文别名 |
更多 |
| 描述 |
Lopinavir是高亲和性HIV蛋白酶抑制剂,Ki为1.3 pM。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 溶解度 |
体外:
DMSO:100 mg / mL(159.03 mM;需要超声波) 体内:1。逐个添加每种溶剂:10%DMSO 40%PEG300 5%吐温-80 45%盐水溶解度:≥2.5mg/ mL(3.98mM);澄清溶液2.逐个添加每种溶剂:10%DMSO 90%玉米油溶解度:≥2.5mg / mL(3.98 mM);明确解决方案
|
||||||||||||
| 储备液 |
|
||||||||||||
| 存储 |
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
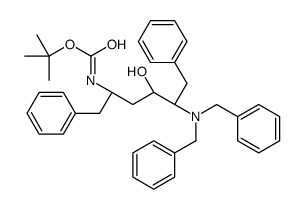
CC1=C(OCC(N[C@@H](CC2=CC=CC=C2)[C@@H](O)C[C@H](CC3=CC=CC=C3)NC([C@H](C(C)C)N4C(NCCC4)=O)=O)=O)C(C)=CC=C1 |
||||||||||||
| 参考文献 |
|
||||||||||||
| 相关活性 小分子 |
Cenicriviroc | 胃酶抑素 A | 依布硒 | 曲西立滨 | 雷特格韦钾盐 | 替拉那韦 | 地拉夫定甲磺酸盐 | 比克替拉韦 | TAK-779 | 白桦脂酸 | 甲磺酸奈非那韦 | 卡替拉韦 | 萘维拉平 | 齐多夫定 |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 924.2±65.0 °C at 760 mmHg |
| 熔点 | 124-127°C |
| 分子式 | C37H48N4O5 |
| 分子量 | 628.801 |
| 闪点 | 512.7±34.3 °C |
| 精确质量 | 628.362488 |
| PSA | 120.00000 |
| LogP | 6.26 |
| 外观性状 | 白色结晶固体 |
| 蒸汽压 | 0.0±0.3 mmHg at 25°C |
| 折射率 | 1.577 |
| 储存条件 | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| 稳定性 | Hygroscopic |
| 水溶解性 | 水溶性:不溶;可溶于:甲醇,乙醇 |
| 分子结构 |
1、 摩尔折射率:179.07 2、 摩尔体积(cm3/mol):541.0 3、 等张比容(90.2K):1433.3 4、 表面张力(dyne/cm):49.2 5、 极化率(10-24cm3):70.99 |
| 更多 |
1.性状:结晶固体 2.熔点(?C):124~127 |
| 洛匹那韦上游产品? 8 | |
|---|---|
|
|
|
|
| 洛匹那韦下游产品? 0 | |
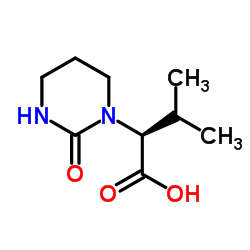
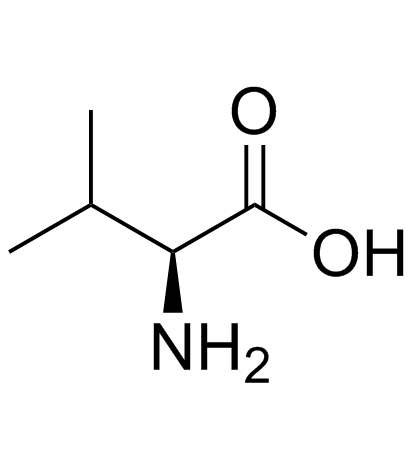
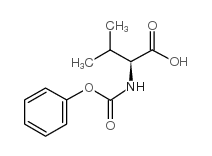
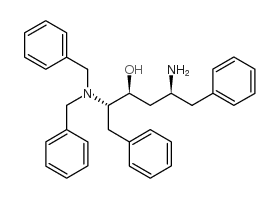
在一定温度下,向二甲亚砜和二氯甲烷的溶液中滴加草酰氯。反应后,加入犖苄氧基羰基氨基丙醇的二氯甲烷溶液,反应一段时间后,滴加三乙胺,搅拌。将其放入冷的10%柠檬酸水溶液中,用乙醚提取。有机层合并,饱和盐水洗涤、干燥、过滤、减压浓缩。经硅胶层析提纯得氨基丙醛衍生物。将其溶于甲醇,加入缬氨酸甲醇盐酸盐、乙酸钠和氰氢硼化钠,在室温搅拌,减压浓缩后溶于乙酸乙酯,用饱和碳酸氢钠洗,洗液用乙酸乙酯提取。有机层合并,用饱和盐水洗,干燥,过滤,减压浓缩。得到的物质用硅胶层析提纯,得缩合还原产物。将该产物进行加氢,然后和等摩尔的1,1-羰基二(1-四氢-嘧啶)在二氯甲烷中反应。所得产物在水和二氧六环中,用氢氧化锂进行水解,盐酸酸化后用乙酸乙酯提取。提取液经干燥、过滤、减压浓缩,得2S-(四氢嘧啶-2-氧-1-基)-3-甲基丁酸。将其和(2S,3S,5S)-2-(2,6-二甲基苯氧乙酰基)氨基-3-羟基-5-氨基-1,6-二苯己烷溶于二甲基甲酰胺,加入1-(3-二甲氨基丙基)-3-乙基碳化二亚胺,反应即得罗匹那韦。
|
ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 42(12) , 3218-24, (1998)
The valine at position 82 (Val 82) in the active site of the human immunodeficiency virus (HIV) protease mutates in response to therapy with the protease inhibitor ritonavir. By using the X-ray crysta...
|
|
|
HIV-1 subtype influences susceptibility and response to monotherapy with the protease inhibitor lopinavir/ritonavir. J. Antimicrob. Chemother. 70(1) , 243-8, (2015)
PI susceptibility results from a complex interplay between protease and Gag proteins, with Gag showing wide variation across HIV-1 subtypes. We explored the impact of pre-treatment susceptibility on t...
|
|
|
Antiretroviral therapy response among HIV-2 infected patients: a systematic review. BMC Infect. Dis. 14 , 461, (2014)
Few data are available on antiretroviral therapy (ART) response among HIV-2 infected patients. We conducted a systematic review on treatment outcomes among HIV-2 infected patients on ART, focusing on ...
|
|
|
1(2H)-Pyrimidineacetamide, N-[(1S,3S,4S)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-, (αS)- |
|
(2S)-N-[(2S,4S,5S)-5-{[(2,6-Dimethylphenoxy)acetyl]amino}-4-hydroxy-1,6-diphenyl-2-hexanyl]-3-methyl-2-(2-oxotetrahydro-1(2H)-pyrimidinyl)butanamide |
|
(2S)-N-[(1S,3S,4S)-1-benzyl-4-{[(2,6-diméthylphénoxy)acétyl]amino}-3-hydroxy-5-phénylpentyl]-3-méthyl-2-(2-oxotétrahydropyrimidin-1(2H)-yl)butanamide |
|
ABT 378 |
|
(2S)-N-[(1S,3S,4S)-1-Benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamid |
|
Lopinavir |
|
Aluviran |
|
1(2H)-pyrimidineacetamide, N-[(1S,3S,4S)-4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-, (αS)- |
|
(aS)-Tetrahydro-N-((aS)-a-((2S,3S)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-a-isopropyl-2-oxo-1(2H)-pyrimidineacetamide |
|
Koletr |
|
(2S)-N-[(1S,3S,4S)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide |
|
(2S)-N-[(2S,4S,5S)-5-{[(2,6-Dimethylphenoxy)acetyl]amino}-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide |
|
ABT 378/r |
|
Koletra |
|
MFCD04973616 |











![N-[(1S,2S,4S)-4-氨基-2-羟基-5-苯基-1-(苯基甲基)戊基]-2-(2,6-二甲基苯氧基)乙酰胺结构式](https://www.chemsrc.com/caspic/013/192725-49-8.png)