This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 描述 |
Vinblastine sulfate是一种针对各种癌症类型的有细胞毒性的生物碱。 长春花碱可抑制微管的形成,抑制nAChR的IC50值为8.9 μM。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 靶点 |
IC50: 8.9 μM(nAChR)[1]
|
||||||||||||
| 体外研究 |
长春碱不会使纺锤体微管解聚,但它有效阻断有丝分裂(例如,HeLa细胞中IC50为0.8 nM),细胞死于细胞凋亡[2]。在NB4细胞中,长春碱产生p53和DNA片段化的改变。长春碱治疗通过诱导细胞凋亡产生Bax / Bcl-2失衡具有抗增殖作用。长春碱处理抑制NFκB表达并抑制NFκB-DNA结合活性,同时维持JNK活化,随后通过半胱天冬酶依赖性途径导致细胞凋亡反应[3]。发现长春碱诱导细胞凋亡,如线粒体膜电位的丧失,细胞色素c和细胞凋亡诱导因子的释放,caspase-9和3的激活以及Poly(ADP-核糖)聚合酶的裂解所证明[4]。 |
||||||||||||
| 体内研究 |
长春碱是一种广泛使用的抗癌药物,具有不良副作用。它与载体分子的结合可能是减少这些副作用的有效策略[5]。 |
||||||||||||
| 溶解度 |
体外:
DMSO:≥44mg / mL(48.40 mM) * “≥”表示可溶,但饱和度未知。
|
||||||||||||
| 储备液 |
|
||||||||||||
| 存储 |
4°C,避光
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
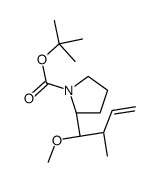
[H][C@@]12C[C@@](C3=C(OC)C=C(N([C@]4([H])[C@@]56[C@]7([H])[C@](C=CCN7CC6)(CC)[C@@H](OC(C)=O)[C@@]4(C(OC)=O)O)C)C5=C3)(C(OC)=O)C(NC8=C9C=CC=C8)=C9CC[N@](C2)C[C@](O)(CC)C1.OS(=O)(O)=O |
||||||||||||
| 参考文献 |
|
||||||||||||
| 相关活性 小分子 |
诺考达唑 | 一甲基澳瑞他汀E | VcMMAE | Mertansine(DM1化合物) | 甲磺酸艾瑞布林 | 埃博霉素 | 酒石酸长春瑞滨 | 埃博霉素B | McMMAF | MMAF盐酸盐 | 伊沙匹隆 | N,BETA,BETA-三甲基-L-苯基丙氨酰基-N-[(1S,2E)-3-羧基-1-(1-甲基乙基)-2-丁烯基]-N,3-二甲基-L-缬氨酰胺 | 雌莫司汀磷酸钠 | 安丝菌素P3 | 考布他丁 A-4 磷酸二钠盐 |
| 密度 | 1.37 g/cm3 |
|---|---|
| 熔点 | 267?°C (dec.)(lit.) |
| 分子式 | C46H60N4O13S |
| 分子量 | 909.053 |
| 精确质量 | 908.387756 |
| PSA | 237.08000 |
| LogP | 4.35970 |
| 外观性状 | 白色粉末 |
| 储存条件 |
2-8?C条件下保存。 |
| 稳定性 |
常温常压下稳定。 |
| 水溶解性 | >=1 g/100 mL at 24.5 ?C |
| 计算化学 |
四、计算化学数据: 1、 氢键供体数量:5 2、 氢键受体数量:16 3、 可旋转化学键数量:10 4、 拓扑分子极性表面积(TPSA):229 5、 重原子数量:64 6、 表面电荷:0 7、 复杂度:1780 8、 同位素原子数量:0 9、 确定原子立构中心数量:9 10、 不确定原子立构中心数量:0 11、 确定化学键立构中心数量:0 12、 不确定化学键立构中心数量:0 13、 共价键单元数量:2 |
| 更多 |
一、物性数据: 1. 熔点(?C):267?C 2. 比旋光度(?): -21.7 ? |
|
|
|
硫酸长春碱毒性英文版
|
| 符号 |


GHS05, GHS07 |
|---|---|
| 信号词 |
Danger |
| 危害声明 |
H302-H315-H318-H335 |
| 警示性声明 |
P280-P301 + P312 + P330-P305 + P351 + P338 + P310 |
| 危害码 (欧洲) |
T:Toxic |
| 风险声明 (欧洲) |
R22;R36/37/38;R41;R61 |
| 安全声明 (欧洲) |
26-36/39-53-45-37/39-36/37/39 |
| 危险品运输编码 | 1544 |
| WGK德国 | 3 |
| RTECS号 | YY8400000 |
| 包装等级 | II |
| 危险类别 | 6.1(a) |
| 海关编码 | 2942000000 |
| 海关编码 | 2942000000 |
|---|
|
Translating clinical findings into knowledge in drug safety evaluation–drug induced liver injury prediction system (DILIps). J. Sci. Ind. Res. 65(10) , 808, (2006)
Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ…
|
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity. Chem. Res. Toxicol. 23 , 1215-22, (2010)
Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals …
|
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury. Drug Metab. Dispos. 38 , 2302-8, (2010)
Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predicti…
|
|
|
(3aR,4R,5S,5aR,10bR,13aR)-4-(acétyloxy)-3a-éthyl-9-[(13S,15S,17S)-17-éthyl-17-hydroxy-13-(méthoxycarbonyl)-1,11-diazatétracyclo[13.3.1.0.0]nonadéca-4(12),5,7,9-tétraén-13-yl]-5-hydroxy-8-m |
|
vinbalastine |
|
Velsar |
|
EINECS 205-606-0 |
|
Vincaleukoblastine, (2′β)-, sulfate (1:1) |
|
VLB |
|
velbe |
|
velban |
|
methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0.0]nonadeca-4(12),5,7,9-tetraen-13-yl]-5-hydr |
|
Vinblastine-d3 |
|
(2′β)-Vincaleukoblastine sulfate (1:1) |
|
(3aR,4R,5S,5aR,10bR,13aR)-4-(acétyloxy)-3a-éthyl-9-[(13S,15S,17S)-17-éthyl-17-hydroxy-13-(méthoxycarbonyl)-1,11-diazatétracyclo[13.3.1.0.0]nonadéca-4(12),5,7,9-tétraén-13-yl]-5-hydroxy-8-méthoxy-6-méthyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate de méthyle sulfate (salt) |
|
Periblastine |
|
vincaleukoblastine, (3β,4′β)-, sulfate (1:1) |
|
VLB Vincaleukoblastine sulfate salt |
|
éthoxy-6-méthyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate de méthyle sulfate (salt) |
|
oxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate sulfate (salt) |
|
(+)-vinblastine |
|
Vinblastine (sulfate) |
|
Vinblastine sulfate |
|
Vincaleukoblastine sulfate salt |
|
Methyl-(3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0.0]nonadeca-4(12),5,7,9-tetraen-13-yl]-5-hydr |
|
Methyl-(3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0.0]nonadeca-4(12),5,7,9-tetraen-13-yl]-5-hydroxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazol-5-carboxylatsulfat(salt) |
|
MFCD00082457 |
|
exal |
|
methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0.0]nonadeca-4(12),5,7,9-tetraen-13-yl]-5-hydroxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate sulfate (salt) |
|
oxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazol-5-carboxylatsulfat(sal
[contact-form-7 id="1574" title="Contact Us"]
|