This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 346.4±15.0 °C at 760 mmHg |
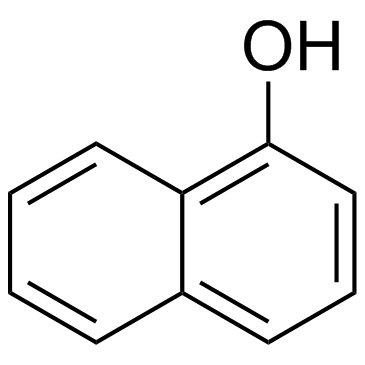
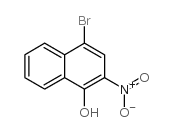
| 分子式 | C10H7BrO |
| 分子量 | 223.066 |
| 闪点 | 163.3±20.4 °C |
| 精确质量 | 221.968018 |
| PSA | 20.23000 |
| LogP | 3.72 |
| 蒸汽压 | 0.0±0.8 mmHg at 25°C |
| 折射率 | 1.705 |
| 符号 |


GHS05, GHS07 |
|---|---|
| 信号词 |
Danger |
| 危害声明 |
H302 + H312-H315-H318-H335 |
| 警示性声明 |
P261-P280-P305 + P351 + P338 |
| 危险品运输编码 | NONH for all modes of transport |
| 海关编码 | 2908199090 |
| 海关编码 | 2908199090 |
|---|---|
| 中文概述 | HS:2908199090 其他酚及酚醇的仅含卤素取代基的衍生物及其盐 增值税率:17.0% 退税率:9.0% 监管条件:无 最惠国关税:5.5% 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | HS: 2908199090. derivatives of polyphenols or phenol-alcohols containing only halogen substituents and their salts. VAT:17.0%. tax rebate rate:9.0%. supervision conditions:None. MFN tariff:5.5%. general tariff:30.0% |
|
Enantioselective synthesis of (+)-estrone exploiting a hydrogen bond-promoted Diels-Alder reaction. J. Org. Chem. 75 , 2718-2721, (2010)
Starting from Dane’s diene and methylcyclopentenedione, (+)-estrone is synthesized along the Quinkert-Dane route in 24% total yield. The key step is an enantioselective Diels-Alder reaction promoted b…
|
|
|
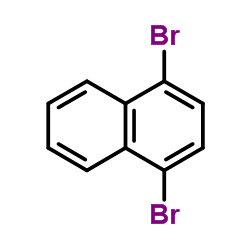
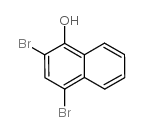
4-bromo-1-hydroxynaphthalene |
|
1-Naphthalenol,4-bromo |
|
4-Bromo-1-naphthol |
|
1-hydroxy-4-bromonaphthalene |
|
4-bromo-1-naphthalenol |
|
4-bromo-naphthalen-1-ol |
|
4-bromonaphthalen-1-ol |
|
1-Bromo-4-hydroxynaphthalene |
|
1-Naphthalenol, 4-bromo- |
|
4-bromonaphthol |
|
4-BROMO-L-NAPHTHOL |