This product was successfully added to cart!
品牌商品推荐
更多 >>商品详情
客户评价
| 中文名 |
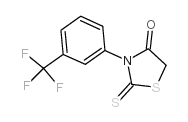
3-[(3-三氟甲基)苯基]-5-[(4-羧基苯基)亚甲基]-2-硫代-4-噻唑烷酮 |
|---|---|
| 英文名 |
CFTRinh 172 |
| 中文别名 |
3-[(3-三氟甲基)苯基]-5-[(4-羧基苯基)亚甲基]-2-硫氧代-4-噻唑烷酮 |
| 英文别名 |
更多 |
| 描述 |
CFTR(inh)-172是高效选择性的CFTR通道阻断剂; 在2分钟内可逆地抑制CFTR短路电流的Ki值为300 nM。 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 相关类别 |
|
||||||||||||
| 靶点 |
Ki: 300 nM (CFTR)[1]
|
||||||||||||
| 体外研究 |
CFTR(inh)-172的抑制在约10分钟内完成(t1 / 2 = 4分钟),并且在t1 / 2约5分钟后反转。 CFTRinh-172在浓度高达100μM后24小时对FRT细胞无毒[1]。 CFTR(inh)-172不改变CFTR单位电导(8 pS),但在Ki =0.6μM时将开放概率降低> 90%。这种效应是由于平均通道关闭时间增加而不改变平均通道开放时间。野生型,G551D和G1349D CFTR中Cl-电流抑制的Ki值约为0.5μM;然而,对于vF508 CFTR,Ki显着降低至0.2μM[2]。 |
||||||||||||
| 体内研究 |
在小鼠中单次腹膜内注射CFTR(inh)-172(250μg/ kg)在6小时内使小肠中霍乱毒素诱导的液体分泌减少超过90%。 CFTR(inh)-172在小鼠模型中在高浓度下是无毒的。 CFTRinh-172显着降低了盐水对照环中的液体分泌,而无活性的CFTRinh-172类似物不抑制液体分泌[1]。 |
||||||||||||
| 溶解度 |
体外:
在DMSO中10mM
|
||||||||||||
| 储备液 |
|
||||||||||||
| 细胞实验 |
将CFTR(inh)-172在DMSO中稀释为10mM储备溶液,并用合适的培养基稀释。产生共表达人野生型CFTR和卤化物指示剂YFP-H148Q的Fischer大鼠甲状腺(FRT)细胞。在用0-1,000μM抑制剂CFTR(inh)-172 [1]孵育细胞后24小时,通过二氢罗丹明方法测定细胞毒性。 |
||||||||||||
| 动物实验 |
小鼠:通过在每天腹膜内注射0-1,000μg/ kg CFTR(inh)-172 [1]后5天测量小鼠的血清化学和血液学来评估动物毒性。 |
||||||||||||
| 存储 |
|
||||||||||||
| 运输 |
室温;可能会有所不同 |
||||||||||||
| SMILES |
S=C(S/1)N(C2=CC=CC(C(F)(F)F)=C2)C(C1=CC3=CC=C(C=C3)C(O)=O)=O |
||||||||||||
| 参考文献 |
|
||||||||||||
| 相关活性 小分子 |
芦马卡托 | 替扎卡托 | GlyH-101 | GLPG1837 | PPQ-102 | BPO-27外消旋体 | IOWH-032 | KM11060 |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 555.7±60.0 °C at 760 mmHg |
| 分子式 | C18H10F3NO3S2 |
| 分子量 | 409.402 |
| 闪点 | 289.9±32.9 °C |
| 精确质量 | 409.005432 |
| PSA | 115.00000 |
| LogP | 4.51 |
| 外观性状 | yellow |
| 蒸汽压 | 0.0±1.6 mmHg at 25°C |
| 折射率 | 1.698 |
| 储存条件 | Store at +4°C |
| 水溶解性 | DMSO: ≥10mg/mL |
| 符号 |


GHS07, GHS09 |
|---|---|
| 信号词 |
Warning |
| 危害声明 |
H315-H317-H319-H335-H410 |
| 警示性声明 |
P261-P273-P280-P305 + P351 + P338-P501 |
| 个人防护装备 |
dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| 危害码 (欧洲) |
Xi,N |
| 风险声明 (欧洲) |
36/37/38-43-50/53 |
| 安全声明 (欧洲) |
26-36/37-60-61 |
| 危险品运输编码 | UN 3077 9 / PGIII |
|
~68%
![5-[(4-羧基苯基)亚甲基]-2-硫氧-3- [(3-三氟甲基)苯基-4-噻唑烷酮结构式](https://www.chemsrc.com/caspic/328/307510-92-5.png)
5-[(4-羧基苯基)亚甲基]…
307510-92-5
|
|
文献:THE REGENTS OF THE UNIVERSITY OF CALIFORNIA Patent: US2011/105565 A1, 2011 ; Location in patent: Page/Page column 28 ; |
|
~%
![5-[(4-羧基苯基)亚甲基]-2-硫氧-3- [(3-三氟甲基)苯基-4-噻唑烷酮结构式](https://www.chemsrc.com/caspic/328/307510-92-5.png)
5-[(4-羧基苯基)亚甲基]…
307510-92-5
|
|
文献:US2011/105565 A1, ; |
|
~%
![5-[(4-羧基苯基)亚甲基]-2-硫氧-3- [(3-三氟甲基)苯基-4-噻唑烷酮结构式](https://www.chemsrc.com/caspic/328/307510-92-5.png)
5-[(4-羧基苯基)亚甲基]…
307510-92-5
|
|
文献:US2011/105565 A1, ; |
| 上游产品? 4 | |
|---|---|
|
|
|
| 下游产品? 0 | |
|
Pseudomonas aeruginosa-induced bleb-niche formation in epithelial cells is independent of actinomyosin contraction and enhanced by loss of cystic fibrosis transmembrane-conductance regulator osmoregulatory function. MBio 6 , e02533, (2015)
The opportunistic pathogen Pseudomonas aeruginosa can infect almost any site in the body but most often targets epithelial cell-lined tissues such as the airways, skin, and the cornea of the eye. A co…
|
|
|
Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS ONE 6 , e22212, (2011)
Evaluation of cystic fibrosis transmembrane conductance regulator (CFTR) functional activity to assess new therapies and define diagnosis of cystic fibrosis (CF) is cumbersome. It is known that leukoc…
|
|
|
Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110 , 1651-1658, (2002)
Secretory diarrhea is the leading cause of infant death in developing countries and a major cause of morbidity in adults. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is requ…
|
|
|
CFTR INHIBITOR-172 |
|
Benzoic acid, 4-[(Z)-[4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-5-thiazolidinylidene]methyl]- |
|
CFTR(inh)-172 |
|
4-[(Z)-{4-Oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene}methyl]benzoic acid |
|
4-[(E)-{4-Oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene}methyl]benzoic acid |
|
Benzoic acid, 4-[(E)-[4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-5-thiazolidinylidene]methyl]- |
|
4-[[4-Oxo-2-thioxo-3-[3-trifluoromethyl)phenyl]-5-thiazolidinylidene]methyl]benzoicacid |
|
CFTRinh-172 |
![5-[(4-羧基苯基)亚甲基]-2-硫氧-3- [(3-三氟甲基)苯基-4-噻唑烷酮](https://www.szhychemical.com/uploads/logo11.png)
![5-[(4-羧基苯基)亚甲基]-2-硫氧-3- [(3-三氟甲基)苯基-4-噻唑烷酮](https://www.szhychemical.com/uploads/bbf0cc66d4ff6421a49a8ceacfd62b78.png)